Cooling A Flask?
So, you have a flask (maybe it contains some chemical) and you want to cool the flask. The obvious answer is to place it in a refrigerator. However, you want to keep it on a magnetic stir plate, which every well stocked kitchen has on hand. You do have a few options, the first is to place the flask and the stir plate into the refrigerator, but then the power cables might block the door… Or, you can use a cooling water bath, like a kitchen ice cream maker, with ice in a container around the thing to be cooled (messy). Unless you have a temperature controlled recirculating water bath, you won’t be able to control the temperature.
There is another option. A Thermoelectric or Peltier Cooling Chip AND our previous temperature controller project. These work by applying electricity to a series of electrical junctions. When the current flows it moves the heat with it – discovered back in 1834. It is moving entropy from one area to another, this change is reflected in the measured temperature – items at lower temperature tend to have less disorder. So, we can use one of these cooling chips for our flask. Now, how big of a chip to buy?
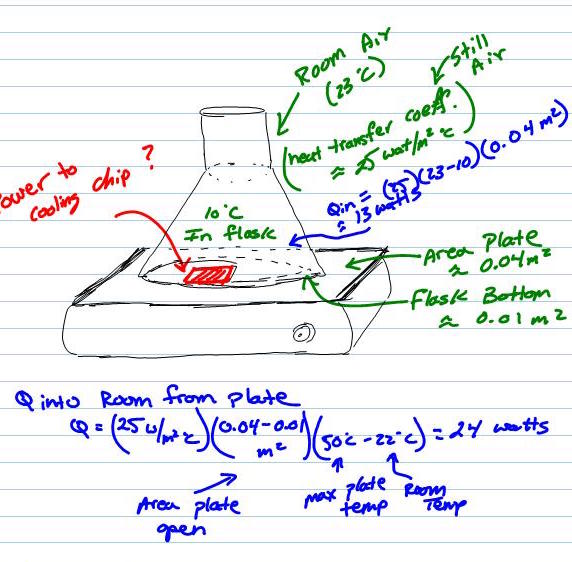
We can apply a bit of heat transfer. Let us assume the room is a nice warm 23°C (or 74°F). There is not wind in the kitchen, so we can say the heat transfer coefficient from the flask wall to the room air is about 25 watts per meter squared per degree centigrade. This number indicates the amount of heat (25 watts) that will exchange to/from the air/flask for each unit of exposed area and degree of temperature difference. So, the bigger the difference in temperature the more heat will want to move between the two items, and more area exposed the more active exchange points exist.
Since we are cooling the flask (lets pick 10°C or 50°F (a refreshing temperature) we can find the amount of heat (Qin) that will be coming into the flask from the warm air. This is simple, make your units work. Qin equals the difference in temperature (23°C – 10°C) times the flask exposed area in squared meters (0.04 m2). Qin is about 13 watts. So, we a chip that can move at least 13 watts.
Now, if we are using the chip to cool the flask the heat needs to go somewhere…. So, we can put the chip under the flask between the stir plate top and the metal/ceramic plate (use a bit of paper towel to form an insulator). Question? is the plate the big enough to handle the heat from the flask with out getting too hot? Let us assume the hottest we want the plate top to get is 50°C (hot bath water). We can do the same calculations, Q to room equals the area of the stir plate exposed to the room air (0.04 sq meters minus the space covered by the flask 0.01 sq meters) times the coefficient of transfer (25 water per sq meter per degree C) times the driving force difference in temperature (50°C – 23°C). This is about 24 watts… We can apply 24 watts to the top of the stir plate before it gets too hot. Good! We are only moving 13 watts from the flask. If this number was too low, the project wouldn’t work or the stir plate would get too hot to be safe. If you have that that problem, you might need cooling fins, a fan, and a bigger stir plate.
Now we know that we can buy a cooling chip that can move at least 13 watts and keep our flask nice and cool. Instructions about building the actual temperature controller are over here in another post.
